Choosing the Right VOC Emission Control Technology
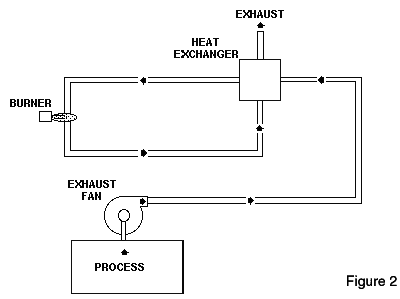
Recuperative Thermal OxidizersThere are a number of recuperative thermal oxidizer designs which can be used to destroy VOCs. The diagram of the thermal oxidizer shown in Figure 2 is a simple design that passes air through an air-to-air heat exchanger to preheat it before entering the burner chamber. In the burner chamber, the process exhaust air is heated to a sufficiently high temperature and held at this temperature with some degree of turbulence to ensure VOC destruction.
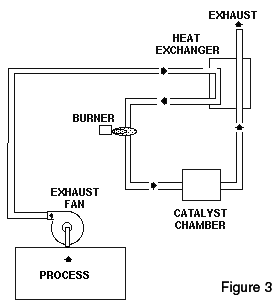
Thermal oxidizers clean emissions by burning or oxidizing them at high temperatures. Typical VOC reduction is 99% or outlet emissions less than 20 mg/Nm ³. Carbon monoxide can be either created (by partial oxidation of VOC) or destroyed (by complete oxidation of the CO to CO2) in a thermal oxidizer, depending upon operating temperature. CO production tends to rise with increasing temperature until it reaches a maximum at about 1200° F (650° C). Then, the CO content tends to decrease rapidly with increasing temperature. At 1400° F (760° C), CO emissions from thermal oxidizers are relatively low. (See Figure 3)
Characteristics
- Moderate-high capital/installation costs
- High operating cost at low solvent loading
- Cleanup rates can be very high (greater than 99%)
- Need high operating temperatures (1380 to 1500° F or 750 - 815° C) to get low CO levels
- NOx formation - especially at operating temperature above 1500° F (815° C)
- High exhaust gas temperatures:
- Secondary energy recovery is possible
- High temperature exhaust stack construction is required
- Quality materials of construction necessary for longevity
Catalytic oxidizers are an alternative to thermal oxidizers for oxidizing gaseous, combustible contaminants into carbon dioxide and water. Their successful operation is limited to a more restricted range of applications than thermal oxidizers; but where applicable, catalytic units offer the potential of significantly lower fuel consumption and operating costs plus reduced CO and NOx emissions. The basic elements of the catalytic unit are a preheat/mixing section, designed to achieve a uniformly preheated and distributed waste stream flow, and the catalyst bed or catalyst matrix, where the majority of the oxidation reactions take place.
The oxidation of most hydrocarbons and carbon monoxide occur rapidly in the range of 300 to 900° F (150 - 480° C) over catalysts. With thermal oxidizers, the oxidation reaction requires a high temperature of 1200 to 1600° F (650 - 815° C) to break the carbon, hydrogen and oxygen bonds.
Besides reduced energy consumption, NOx emissions from catalytic units are very low because of the lower oxidation temperatures being used, as well as the lower burner firing rates. In addition, the oxidizing nature of the catalyst results in very low CO emissions. However, there are trade-offs involved to gain these advantages. Catalytic oxidizers can be subject to masking agents or poisons that inhibit the effectiveness of the catalyst.
Catalytic systems are limited to applications in which the waste stream has negligible particulate loading and/or "poisons" which can reduce the effectiveness of the catalyst. These poisons are primarily silicon and phosphorus which coat the catalyst; halogens such as chlorine which directly attack the active metals converting them to an inactive form; and sulfur which inhibits the activity of some catalysts. The oxidation activity of the catalyst can also be reduced by the loss of active components through attrition, deposition of unreacted VOC (coking) onto the catalyst surfaces, or sintering of the catalyst (collapse of the catalyst structure caused by high temperatures).
(See Figure 3)